Abstract
Background. Idiopathic pure red cell aplasia (PRCA) and secondary PRCA not responding to the treatment of the underlying diseases are generally thought be immune-mediated and treated by immunosuppressive therapy. We previously conducted the PRCA2004/2006 study and reported that poor response to induction therapy and relapse of anemia were associated with death. Principal causes of death were infections and organ failure. Based on the literatures, idiopathic PRCA may represents the prodrome to myelodysplastic syndromes. Theoretically, there are two potential mechanisms of unresponsiveness to immunosuppression; the clonal hematopoiesis by the stem/progenitor cells that have undergone somatic mutations during disease progression of PRCA and the clonal changes of auto-aggressive lymphocytes reacting against erythroid progenitors.
Objectives. In this study, we investigated the somatic mutations of myeloid malignancy-associated genes in acquired PRCA in order to determine how often clonal hematopoiesis is detected in this disorder.
Materials and Methods. This study included 23 patients with chronic acquired PRCA (12 idiopathic, 7 thymoma-, 2 LGL leukemia- and 2 systemic lupus erythematosus-associated PRCA) with a median age of 62 (range: 40-62). Disease status was varying. After obtaining informed consent, heparinized blood was drawn and mononuclear cells were separated by density gradient centrifugation. Extracted genomic DNA samples were subjected to targeted sequencing for 54 myeloid malignancy-associated genes using a TruSight Myeloid Sequencing Panel kit according to the manufacturer's instruction (Illumina). Criteria for the significant somatic mutations of myeloid malignancy-associated genes in the present study were as follows: potential functional consequences such as missense, nonsense or frameshift mutations; exclusion of previously reported SNPs; being recurrently detected in two sequencing runs; variant allele frequency (VAF) exceeding 0.02 and less than 0.40. The institutional review board approved the experimental protocol.
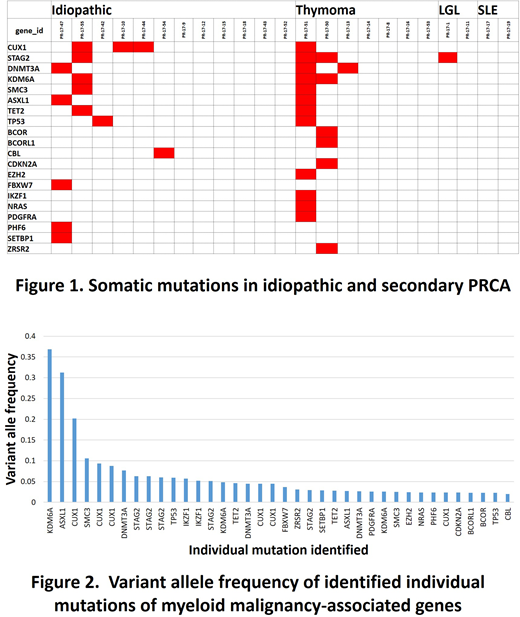
Results. We detected some mutations of the targeted genes in 20 out of 23 patients, and the somatic mutations defined by the criteria mentioned above were found in 10 patients including 6 idiopathic, 3 thymoma-associated and one LGL leukemia-associated PRCA (Fig. 1). These 10 patients had 38 distinct mutations in 20 genes. Variant allele frequencies were 0.02 to 0.37 (median, 0.04; average, 0.06, Fig. 2). Four patients had more than one mutated genes and multiple genes were mutated in some patients (Fig. 1). The most frequently mutated gene was CUX1 that was found in four patients, and STAG2, DNMT3A, KDM6A, SMC3A, ASXL1, TET2 and TP53 were mutated in more than one patient.
Discussion/Conclusion. This study demonstrated that myeloid malignancy-associated genes were somatically mutated in 43% of acquired chronic PRCA patients. This figure appears to exceed the prevalence rate of clonal hematopoiesis of indeterminate potential (CHIP) in the general population with the age of 60s. These mutations were presumably carried by monocytes, because DNA samples were prepared from PBMCs in this study cohort. Profiles of mutated genes in PRCA appear to be different from those of aplastic anemia that were previously reported by other groups. It is yet to be known whether this could result from the different nature of both diseases, or the difference in the experimental protocols. Our findings strongly encourage conducting a prospective study to confirm our observation and clarify the diagnostic and predictive values of somatic mutations of myeloid malignancy-associated genes in acquired PRCA. This project is ongoing in collaboration with the prospective cohort study PRCA2016 being conducted in Japan.
Nakao:Kyowa Hakko Kirin Co., Ltd.: Honoraria; Novartis: Honoraria; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. Matsuda:GlaxoSmithKline K.K.: Honoraria; Novartis Pharma K. K.: Honoraria; Chugai Pharmaceutical Co, Ltd.: Honoraria; Kyowa Hakko Kirin Co, Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Celgene Corporation: Honoraria; Alexion Pharmaceuticals, Inc.: Honoraria; Sanofi K.K.: Honoraria; Beckman Coulter K.K.: Honoraria. Mitani:Kyowa Hakko Kirin Co., Ltd.: Consultancy, Research Funding, Speakers Bureau; Bristol-Myesr Squibb: Research Funding, Speakers Bureau; Celgene: Speakers Bureau; Chugai: Research Funding; Astellas: Research Funding; Sumitomo Dainippon: Research Funding; Novartis: Research Funding; Toyama Chemical: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.